New Cancer-Busting Protein Brings Hope in Cancer Immunotherapy
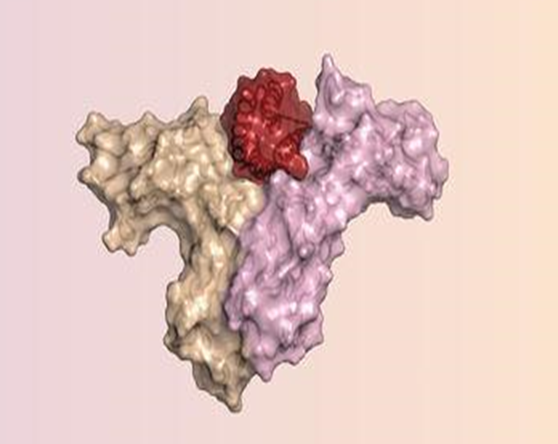
The red portion is the redesigned protein that binds the immune cell receptors to the grey and purple portions. This boosts up immune activity that can kill the cancer cells. Image Courtesy: Science Magazine
There is good news for those who are looking up to Cancer Immunotherapy, especially in the cases of kidney and skin cancers. Interleukin-2 (IL-2) is a protein that helps fighting cancer by boosting immunity. But the dose at which IL-2 used to be administered in cancer patients could lead to life-threatening side effects. However, now, researchers have designed a new protein that confers immune boosting capability to patients like that of IL-2, but without having those dangerous side effects. This redesigned protein has so far been tested in animals, but it may soon enter human trials.
How IL-2 Functions
IL-2 belongs to the class of molecules called the cytokines, which ramp up immune response of the body in response to outside invaders. This protein binds to the T cells, a kind of white blood cell. White blood cells are the immune cells that fight against outside invaders. In the T cell, there are receptors to which IL-2 bind—the IL-2β and the IL-2γ receptors. Binding of IL-2 to these receptors activate the T cells and direct it to fight for the body’s defence.
But there are certain cells among the white blood cells that have a third kind of receptor to which IL-2 can bind to. This is named as IL-2α. The presence of this receptor in certain cells creates a deadly condition. When IL-2 is administered it binds to all the three types of receptors with equal affinity. The cells having the IL-2α receptors can cause the immune response dampen when IL-2 binds to it. Moreover, this receptor is also present in cells in blood vessels. Binding of IL-2 to this receptor in the blood vessel cells can lead those vessels to leak. This can cause a fatal reaction.
Redesigning of IL-2 to Avoid the Dangerous Side Effects
Daniel Adriano Silva Manzano, the lead author of the new study and a biochemist of the University of Washington, US, said, “People have tried for 30 years to alter IL-2 to make it safer and more effective, but IL-2 is unstable and stops working when it loses its normal 3D shape, and many mutations destabilise the structure further.”
Silva Manzano and his team started with studying the atomic maps of IL-2 binding with the desirable β and γ receptors along with the undesirable α receptor. The IL-2 molecule is a single long chain of amino acids that folds up to the 3D active form—consisting of spirals called the alpha helix. The alpha helices are held together in a bundle by a series of loops. At the bottom of this bundle, there are two sites that have the β and γ receptors. Again, portions of one of the helices and two loops at the top of the protein bind the α receptor.
Silva and his team, through computer simulations, designed a protein that mimics the structure and function of IL-2 while maintaining the needed interactions with β and γ receptors but eliminate the portion that binds to α receptors. They produced 40 such designs. After primary analysis, they synthesised and tested 22 out of the 40 initially designed proteins.
Finally, the researchers zeroed in on a version they named as the Neo-2/15. Lab studies revealed that synthesised Neo-2/15 tightly binds to β and γ receptors, but not to α receptors. This has been tested on a mouse model of colon cancer and melanoma. Neo-2/15 strongly inhibited the tumour growth in the mice and also inhibited tumours in some of them. But the specifically designed protein did not elicit the deadly side effects as seen in the case of IL-2.
Get the latest reports & analysis with people's perspective on Protests, movements & deep analytical videos, discussions of the current affairs in your Telegram app. Subscribe to NewsClick's Telegram channel & get Real-Time updates on stories, as they get published on our website.