Chain-Melted State: The Strange State of Matter

Image Courtesy: Science Alert. Image for representational use only.
Finding more about the nature of states that matters can exist in, scientists now have found that some matters can also exist in two states at the same time, provided some particular conditions are met with. Potassium, a metal, when subjected to extreme pressure and extreme temperature, can exist in both solid and molten state. As it has been published in the Proceedings of National Academy of Sciences (PNAS) on April 11, this state of potassium is named as the Melted Chain State.
Scientists have shown previously that putting under extreme conditions, simple metals can behave strangely. For instance, the conducting metal sodium becomes insulator when put under high pressure. Lithium, under high pressure and low temperature, becomes a superconductor.
On potassium also, experiments have been conducted. The Physical Review B paper published in 2015 showed that the atoms of potassium under high pressure organize themselves into a complex arrangement. The atoms form five tubes, and get organised in a square. Four tubes lie at the four corners of the square with the fifth tube at the center of the square and four chains of atoms bound between them. This arrangement is shown below schematically—four chains of atoms at the four corners of the square and the fifth chain at the middle of the square. They are interconnected by chains of atoms.
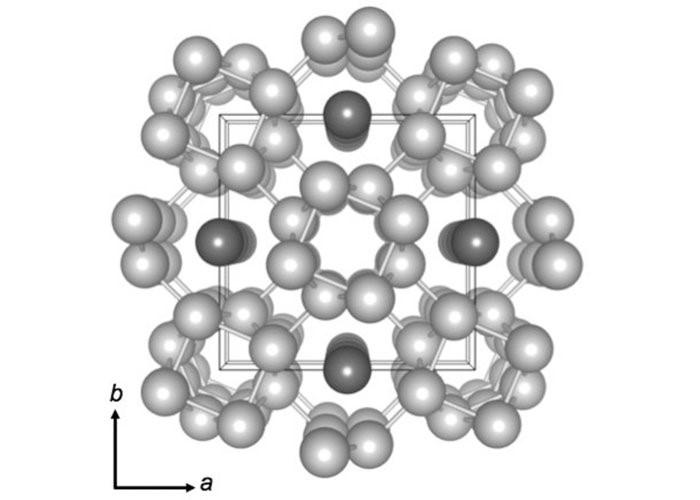
Image of Atomic arrangement of Potassium under high pressure. Image courtesy: Sciencealert.
Now, when the heat is applied, the chains disappear. The researchers call this the Chain Melting State. It was thought that the chain melting state is achieved by the transition of the potassium chain from an ordered to a disordered state.
The latest PNAS study was designed to understand why potassium under high pressure and temperature had the melted chain state. The researchers designed powerful computer simulations to observe how the atoms of potassium under extreme conditions behave. They, in total, studied the behavior of 20,000 potassium atoms under these conditions.
Under normal condition, potassium has a pretty simple configuration—a basic crystal structure. But when the pressure and temperature are high enough, the potassium atoms rearrange themselves in interlinked chains and lattices.
When the temperature is within the range of 400-800 kelvin, the interactions between the lattice atoms are strong and they remain an ordered solid. But, at the same time, the chains melt into a disordered liquid state.
The team of the researchers believes that several other metals like sodium and bismuth could also exist in the chain melted state if the right conditions are met with.
Physicist Andreas Hermann of University of Edinburgh, who is a lead author of the study, said, “We have shown that this unusual but stable state is part solid and part liquid. Recreating this unusual state in other materials could have all kinds of applications.”
Get the latest reports & analysis with people's perspective on Protests, movements & deep analytical videos, discussions of the current affairs in your Telegram app. Subscribe to NewsClick's Telegram channel & get Real-Time updates on stories, as they get published on our website.















